Quality is our cornerstone
In a world with infinite variables, simplicity and reliability are key
That’s why we have a no-compromise approach to quality for every product. For you, this means products you can rely on for better success rates that are safe for the patient.
Our products undergo thorough quality testing before being released, to ensure consistent quality for your piece of mind.
Our focus on quality at every level of our operations is audited and confirmed by our notified bodies, that delivers quality certificates.
Feedback from customers is crucial. We welcome your thoughts and suggestions as you are the source of inspiration for improvements to our products. Click here to contact us.
EC, ISO Certifications and Licences
Quality Management System – ISO 13485:2016 – Certificate: FM 72267
Quality Management System – ISO 13485:2016 – Certificate: MDSAP 681109
ORIGIO EC CERTIFICATES
ORIGIO ISO CERTIFICATES
ORIGIO a/s, ISO 13485: DGM-510
ORIGIO B.V., ISO 13485:2016 & EN ISO 13485:2016 BSi – MD605567
RI EC CERTIFICATES
Research Instruments Ltd EU CE Certificate: CE 735275
RI ISO CERTIFICATES
TPC EC CERTIFICATES
TPC EC Certificate: MRA Q00053
TPC ISO CERTIFICATES
Quality Management System Certificate ISO 13485 – 2014/028080
TPC CONFORMITY ASSESSMENT CERTIFICATE
Quality Management System – ISO 13485:2016 – Certificate FM 703204
Quality Management System – ISO 13485:2016 – Certificate MDSAP 685801
CooperGenomics Certification and Licenses
Based on location, CooperGenomics laboratories hold various global licenses, certifications, and accreditations, serving as a safe and reliable laboratory partner. We are proud to be certified, licensed, and/or accredited by the following:
New Jersey Laboratory
Quality Testing Across Our Brands
TPC
Ultra Clean Manufacturing
All manufacturing and packaging is carried out in a state-of-the-art cleanroom which is certified to ISO 14644 class 7. Special filters remove particles, bacteria, VOCs and other airborne and volatile contaminants.
The cleanroom is maintained spotlessly clean and undergoes regular microbiological screening.
To minimise contamination, personnel wear a full set of cleanroom garments – coverall, hood, overshoes, gloves and mask. Gloves are rinsed and decontaminated regularly during use, and undergo regular microbiological screening.
Only ultrapure water is used: > 18.2 MΩ per cm, TOC < 10 ppb, bacteria < 1 CFU per 500 ml, endotoxins < 0.03 EU per ml.
These measures maintain an ultraclean manufacturing environment which ensures ultraclean, sterile pipettes.
Quality Assurance
We aim to provide the highest quality pipettes and microtools through rigorous quality control and quality assurance with certification to ISO 13485, excellent customer service and technical support.
Manufacturing Quality Control
Every pipette is checked and measured repeatedly during manufacture and inspected by an experienced technician before packaging to ensure that they are intact, clean and comply with specifications.
Strict rejection criteria are used during all stages of manufacturing. Pipettes which do not strictly conform to specifications and standards of cleanliness are rejected without compromise.
We discard what other manufacturers sometimes package and sell.
Batch Quality Assurance
Every batch is tested by an independent laboratory using a validated mouse embryo assay (MEA).
To maximise sensitivity: (i) one-cell zygotes are cultured to fully expanded blastocysts (FEB); (ii) a simple culture
medium is used to slightly ‘stress’ the embryos; (iii) protein-free culture medium is used so that toxicity is not
masked by protein; (iv) embryos are directly exposed to pipette tips for the entire 96 hour culture period.
Every batch is also tested by an experienced embryologist using a micromanipulator set up to ensure that pipettes are ultraclean, non-sticky and function smoothly and efficiently under routine micromanipulation conditions.
Batch quality certificates can be downloaded here.
Other Quality Assurance Procedures
The cleanroom is maintained spotlessly clean, undergoes regular microbiological screening and is certified to ISO 14644 class 7.
Water systems are rigorously maintained, tested regularly for bacteria and endotoxins, and certified annually by the manufacturer.
Packaging materials are thoroughly inspected and tested to ensure cleanliness and sterility.
Packaging and sterilisation processes are fully validated to a Sterilization Assurance Level (SAL) of 10
Guarantee of Quality
If any of our pipettes have manufacturing defects, we will gladly replace them free of charge.
Research Instruments
Sterility and Biocompatibility Testing
RI’s range of consumables must pass stringent testing and inspection criteria to ensure that you receive the most consistent and high quality product.
Mouse Embryo Assay (MEA) for embryo toxicity
Each sterility batch of finished product is tested by an independent laboratory using a 1-cell stage mouse embryo toxicity test. Embryos are also assessed at critical development points during the MEA to ensure maximum test sensitivity. Non-embryotoxic proven by Mouse Embryo Assay (MEA), Pass Level: Day 2 ≥80% 2-cells, Day 6 ≥80% blastocysts.
Limulus Amoebocyte Lysate (LAL) for endotoxins
Each sterility batch of finished product is tested for bacterial endotoxins. Our pass criteria is < 20Eu per device.
Human Sperm Survival Assay (HSSA)
Each sterilisation batch of finished injection pipettes within the RI Pipette range undergoes a sperm motility bio assay. To pass the HSSA test, the motility of sperm should be ≥ 70% after 24 hours of sample preparation.
Sterilisation
Where applicable, products are sterilised by an approved gamma irradiation facility meeting ISO 11137 standards to a sterility assurance level (SAL) of 10-6. The shelf life of our pouch packed products is 3 years and for our blister packed products, 5 years.
Device Approval
All RI medical devices carry the CE mark.
The CE mark shows that RI’s products comply with the Essential Safety Requirements of the European Medical Device Directive (93/42/EEC) as amended.
Many of our medical devices also have USA FDA market clearance, Health Canada medical device licenses and certification in Taiwan and China.
We are continuously working to expand our registration with the relevant medical device authorities across the world.
ORIGIO
ART Media
Monitoring and quality control testing cover all processes from the receipt of raw materials to the final product, including product development.
The final products are tested with a combination of the following QC tests according to our specifications:
pH test: Ph. Eur., USP current edition
Osmolality test: Ph. Eur., USP current edition
Mouse Embryo Assay (MEA): Embryos are cultured in test medium and control medium for 72 hours (for 2-cell) or 96 hours (for 1-cell) respectively. ORIGIO requires a minimum of 80% formation rate to expanded blastocysts. All relevant final products are tested by a MEA test.
Endotoxin LAL test: Ph.Eur., USP current edition
Sterility test: Ph.Eur., USP current edition. All products are sterile filtered and each batch is tested for sterility.
Sperm Survival Test: This test is intended to demonstrate the absence of substances that will reduce the viability of spermatozoa. The Sperm Survival Test is based on CASA (Computer Assisted Semen Analysis). Determination of VAP (average path velocity), VSL (straight-line velocity) and VCL (curvilinear velocity) is used to calculate the concentration of motile spermatozoa. After incubation in media the survival rate must be ≥ 80%.
Sperm Immobilization Test: Sperm with normal motility characteristics are mixed with the media. To pass the test, VSL of the sperm suspended in the media must be reduced by more than 70%.
Note: The results from each batch are stated on a certificate of Analysis, which is available here.
Humagen Pipets
Mouse Embryo Assay (MEA): Each master lot of micropipet glass and Pasteur pipets are tested for toxicity in a one-cell mouse embryo assay (MEA). The percentage of embryos exposed to the micropipets or Pasteur pipets that develop to the blastocyst stage are compared to control embryos. The embryos are assessed at 96 hours of culture. The product and control is assessed and the product will be considered non-toxic if the percentage of blastocysts exposed to the product is greater than or within 10% of the control percentage. Seventy percent or more of the control embryos must reach the blastocyst stage for the test to be considered valid.
Endotoxin LAL test: Each master lot of micropipet glass and Pasteur pipets is endotoxin tested using the Limulus Amebocyte Lysate (LAL) assay. Each lot must be less than 20 endotoxin units per device to pass. Specific testing information and results can be found on the Certificate of Analysis provided with each lot of micropipets and Pasteur Pipets. In addition, independent laboratory tests of packaging, sterilization and expiration date validation are conducted on all products during design and upon changes to materials or production.
Note: The results from each batch are stated on a certificate of Analysis, which is available here.
Needles and Catheters
Mouse Embryo Assay (MEA)
Endotoxin LAL test
Note: The results from each batch are stated on a certificate of Analysis, which is available here.
Cryopette®, Stripper® tips
Mouse Embryo Assay (MEA)
Sterility test
Endotoxin LAL test
Note: The results from each batch are stated on a certificate of Analysis, which is available here.
Quality documents
ORIGIO has obtained CE markings for a range of its products. The CE mark covers most of our ORIGIO products including among others: media, pipets, the Stripper® and Cryopette®.
The CE mark ensures that the products comply with the relevant European health and safety legislation. IVF products are covered by the Medical Device legislation and therefore ORIGIO’s products do comply with the Essential Safety Requirements in the European Medical Device Directive (93/42/EEC).
Our Global Laboratory and Production Facilities
Måløv, Denmark

ORIGIO Liquid media are manufactured at this site. It is DS/EN ISO ISO 13485:2012 and DS/EN ISO 9001:2008 certified. Media are mixed, sterile filtered and aseptically filled in cleanrooms that are classified ISO 5-8.
Alajuela, Costa Rica

Our production facility for Needles and Catheters and Humagen pipettes
Falmouth, United Kingdom

Hardware and consumables from Research Instruments (RI) are manufactured and packaged at this site. It holds certification to EN ISO 13485:2012 (including CMDCAS) and Medical Device Directive 93/42/EEC as amended.
Hilvarenbeek, The Netherlands

ORIGIO needles, catheters and suction pump are produced at this stand-alone world class development site which is registered ISO 13485:2003 & EN ISO 13485:2012.
CooperGenomics, North America
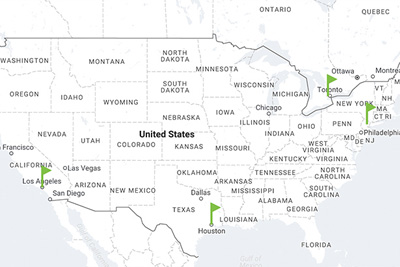
Laboratory locations in North America
- Houston, TX
- Livingston, NJ
- Los Angeles, CA
- Toronto, ON
CooperGenomics, United Kingdom
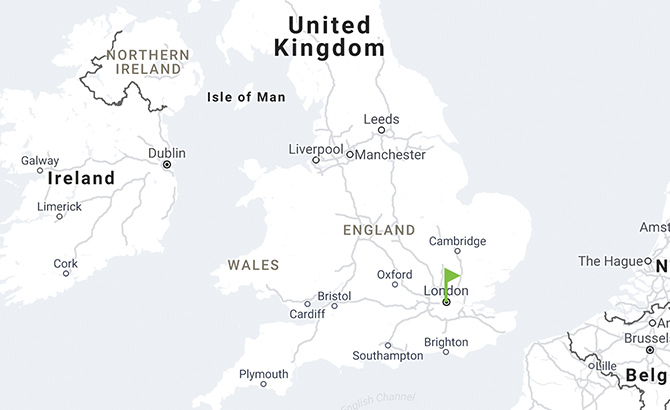
Laboratory locations in the United Kingdom
- London, England

